Start and Stay
with BRIVIACT for
Pediatric Patients
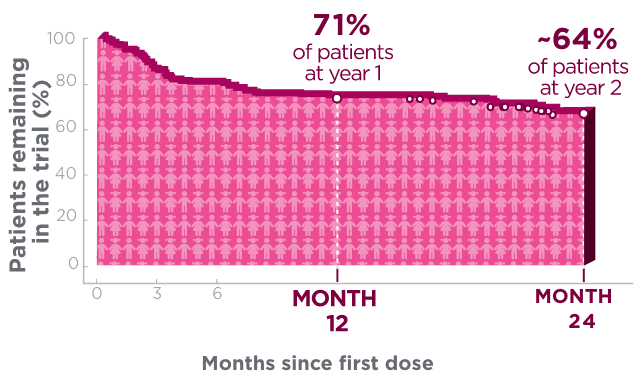
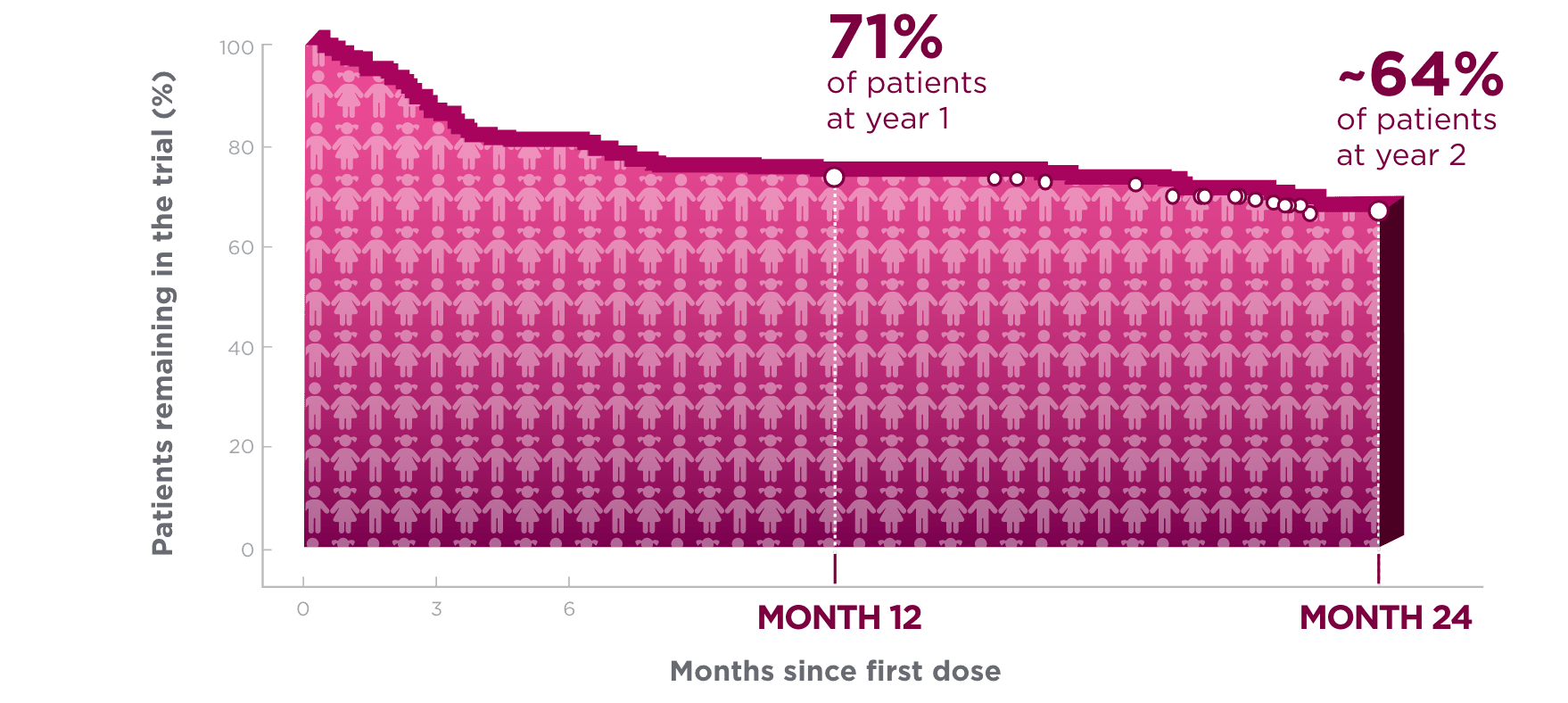
One year after starting treatment, ~7 in 10 pediatric patients remained on BRIVIACT1
Kaplan-Meier curve of pediatric patients remaining on
treatment in a pooled study1*†
*
Kaplan-Meier estimates of the percentage of subjects completing only the specified duration of treatment with BRIVIACT regardless of any discontinuation reason.1
†
Each circle represents at least one censored event.1
Two years after starting treatment, an estimated
6 in 10 pediatric patients remained on BRIVIACT1
- This analysis was focused on time to discontinuation among patients with focal seizures (n=168) aged ≥1 month to <17 years, uncontrolled by 1-3 prior ASMs1,2
-
These retention data are based on Kaplan-Meier analyses of how long patients remained on BRIVIACT.
Conclusions of long-term efficacy or safety should not be drawn based on this data
- Pooled data from Phase 2a and Phase 3a open-label trials of BRIVIACT
- Of the 219 patients, 168 had focal seizures
- After dose adjustment, patients received BRIVIACT 1 to 5 mg/kg/day (maximum 200 mg/day)
ASM=antiseizure medication.
References: 1. Data on file. UCB, Inc. 2. Patel AD, Badalamenti V, Gasalla T, Elmoufti S, Elshoff JP. Safety and tolerability of adjunctive brivaracetam in children with focal seizures: interim analysis of pooled data from two open-label trials. Eur J Paediatr Neurol. 2020;25:68-76. doi:10.1016/j.ejpn.2019.11.007 3. Liu E, Dilley D, McDonough B, Stockis A, Daniels T. Safety and tolerability of adjunctive brivaracetam in pediatric patients <16 years with epilepsy: an open-label trial. Paediatr Drugs. 2019;21(4):291-301. doi:10.1007/s40272-019-00332-y