BEHAVIORAL & COGNITIVE
Stability in Pediatric
Patients
Behavioral and cognitive stability with BRIVIACT:
Pediatric measures were generally unchanged from baseline1
Based on 2 established neurological measurement tools
Parents or caregivers completed the BRIEF® and Achenbach Child Behavior Checklist (CBCL) surveys at regular intervals during the long-term, Phase 3a, follow-up study. The version appropriate for the patient’s age at each visit was completed.1,2
Executive function and self-regulation categories remained stable for the majority of school-aged children (aged 5 to ≤17 years), relative to baseline1
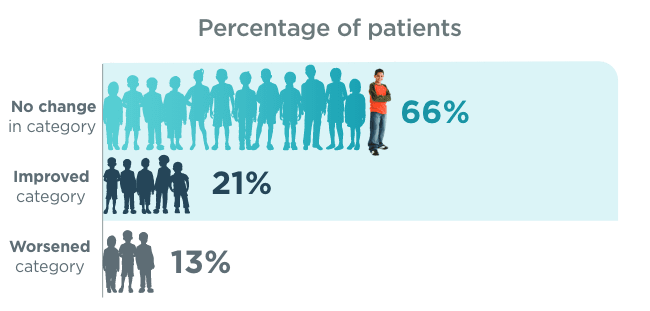
BRIEF® Global Executive Composite score category changes (n=92)1*
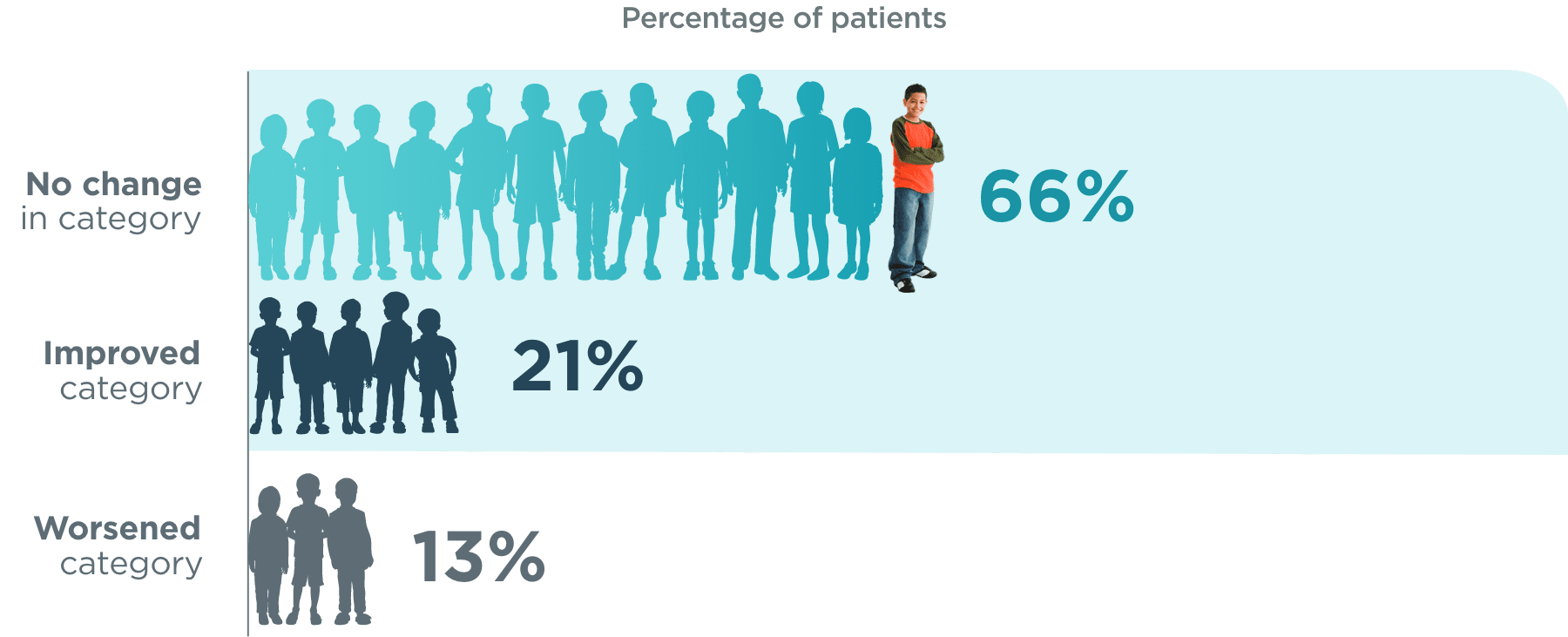
*
T-score categories were defined as “normal” (0 to <50), “borderline” (50 to <65), and “clinically significant” (≥65).1
BRIEF®=Behavior Rating Inventory of Executive Function.1
~9 out of 10 patients aged 5 to ≤17
did not worsen in category from baseline1
Emotional, behavioral, and social problem categories remained stable among the majority of school-aged children (aged 6 to <17 years), relative to baseline1
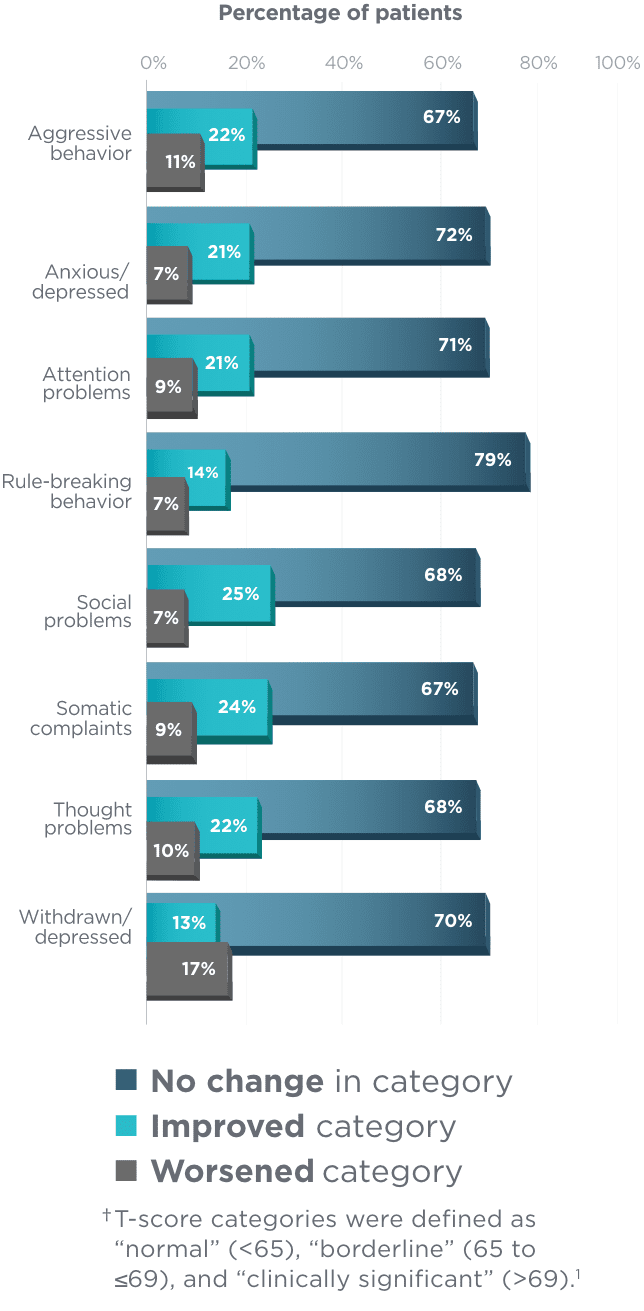
Shift in CBCL T-score categories (n=127)1†
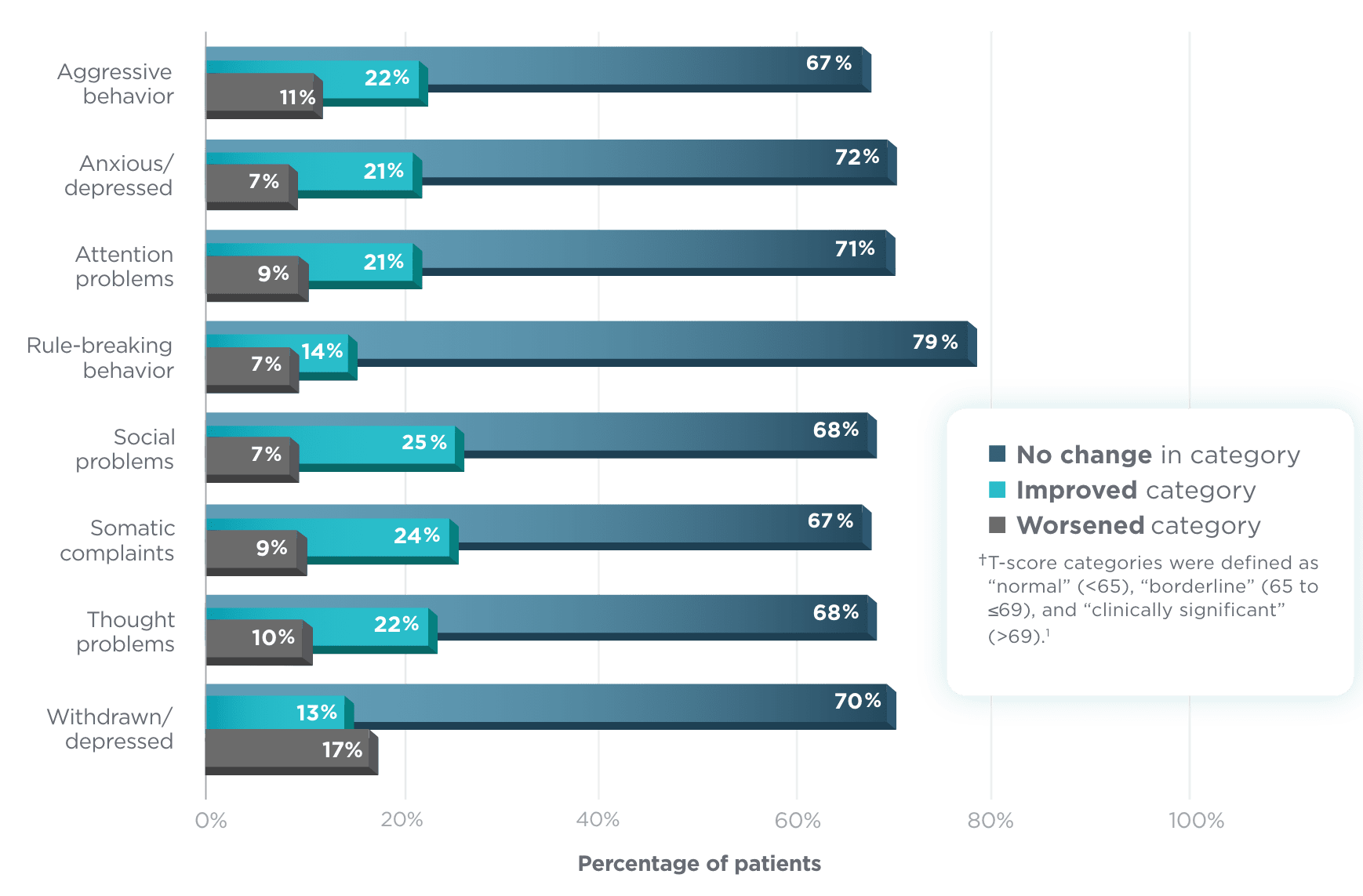
For all child behavior rating scales, the
majority of patients aged 6 to <17 did not worsen
in categories from baseline1
A similar trend was observed among patients aged 1.5 months
to 5 years who were assessed by a separate CBCL.1
Study design for evaluating BRIEF® and Achenbach CBCL
- Pooled interim analysis from Phase 2a and 3a open-label trials
- Included pediatric patients 1 month to <17 years (N=219) uncontrolled by 1 to 3 ASMs
- Of the 219 patients, 168 had focal seizures
- ASM changes were permitted over the duration of the long-term follow-up study
- Additional factors may influence measures of behavior and cognition
ASM=antiseizure medication.
PSYCHIATRIC ADVERSE REACTIONS: BRIVIACT causes psychiatric adverse reactions, including non-psychotic and psychotic symptoms. These events were reported in approximately 13% of adult patients taking at least 50 mg per day of BRIVIACT compared to 8% of adult patients taking placebo. A total of 1.7% of adult patients taking BRIVIACT discontinued treatment due to psychiatric reactions compared to 1.3% of patients taking placebo. Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults. Advise patients to report these symptoms immediately to a healthcare provider.3
References: 1. Data on file. UCB, Inc. 2. Patel AD, Badalamenti V, Gasalla T, Elmoufti S, Elshoff JP. Safety and tolerability of adjunctive brivaracetam in children with focal seizures: interim analysis of pooled data from two open-label trials. Eur J Paediatr Neurol. 2020;25:68-76. doi:10.1016/j.ejpn.2019.11.007 3. BRIVIACT® (brivaracetam) prescribing information. Smyrna, GA: UCB, Inc.